Federal lawsuit filed over surgical procedures
A Delaware legal firm has filed a federal lawsuit over surgical patients who contracted tuberculosis from the bone-graft implement FiberCel.
Plaintiffs from four states, including one in Delaware, have joined the suit against manufacturer Aziyo and distributor Medtronic for personal injuries they say they have sustained from tuberculosis-tainted FiberCel.
Keith Donovan, an attorney for Wilmington-based Morris James, which along with Philadelphia-based firm Saltz Mongeluzzi and Bendesky is bringing the cases, said he expects many more people to come forward with infected bone grafts. He said to his knowledge, more than 100 people had tuberculosis-infected grafts put in them.
FiberCel is made from human cadaver tissue; it is engineered to be like natural tissue and used as a bone void filler in orthopedic and spinal procedures. Aziyo, which makes FiberCel, is based in Wilmington, while distributor Medtronic is based in Minneapolis. Aziyo created the product and Medtronic sold it. The product is marketed for use in orthopedic and reconstructive bone-graft procedures, a surgery where transplanted bone is used to repair damaged or diseased bones.
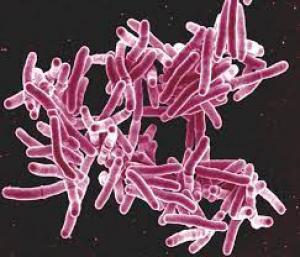
On June 2, the U.S. Food and Drug Administration issued an urgent recall of FiberCel after Aziyo received reports of patients testing positive for tuberculosis and post-surgical infections in patients who had received spinal procedures. Donovan said one of Aziyo’s cadavers was found to have had tuberculosis, a bacterial infection which can occur in any part of the body, although it more often than not affects the lungs. Symptoms include coughing, weight loss or fever, and the disease can be fatal.
Donovan said the recalled lot contained 154 units of infected FiberCel delivered to 20 states. According to legal filings, Aziyo has acknowledged that at least one hospital has reported post-surgical infections in seven of 23 patients who received infected FiberCel.
Donovan said the first lawsuit was filed by Richard Williams, an existing client who had cervical fusion neck surgery with an infected graft. Donovan said Williams later began showing symptoms of tuberculosis.
While he does not know what kind of testing Aziyo did on the cadaver that turned out to be infected, Donovan said the product was harvested, frozen and then sold to hospitals. Other clients include Deborah Rice, a Florida woman who tested positive for tuberculosis after a spinal surgery in March. Also among the clients is Jean Georges, a Newark man who was infected with tuberculosis after a back surgery; according to attorneys from Morris James, Georges had to undergo a second surgery to remove and replace spinal fluid.
His attorney, Matthew Fogg, said, “There is no doubt that Mr. Georges contracted tuberculosis as a direct result of the contaminated, recalled Aziyo FiberCel implant used in his surgery. His life has been a living hell, and he is filing a lawsuit to ensure that those responsible for his pain and suffering are held fully accountable.”
Donovan said at this point, Morris James has 12 clients in five states, and more are forthcoming. He said more than money, those clients want to know how this could have happened and want to ensure that it doesn’t happen again. While the litigation is in its very early stages, Donovan said he does not think the cases will morph into a class-action lawsuit, given the difficulties presented by the wide-ranging jurisdiction and the lengthy process of class certification.
“We do expect more of these across the country,” he said.
Ryan Mavity covers Milton and the court system. He is married to Rachel Swick Mavity and has two kids, Alex and Jane. Ryan started with the Cape Gazette all the way back in February 2007, previously covering the City of Rehoboth Beach. A native of Easton, Md. and graduate of Towson University, Ryan enjoys watching the Baltimore Ravens, Washington Capitals and Baltimore Orioles in his spare time.